Safety
TMS is a safe, non-invasive procedure that is FDA-approved to treat depression, and studies have also shown that TMS is effective in treating other neurological conditions as well. Indeed, it is the least invasive treatment option for depression with the fewest potential side-effects, compared to other treatments like oral antidepressants (which may have significant side effects) and ECT (a more invasive procedure that may have significant side effects).
TMS is performed by a trained TMS technician and is also supervised and performed by the prescribing psychiatric professional. The treatment takes place at our offices, treatment sessions last under 30 minutes, and patients are able to drive themselves home after a treatment session. There are few risks associated with TMS treatment. There is no evidence of damage or brain damage or degeneration. On the contrary, TMS is a neuromodulation therapy that aids in neurogenesis (i.e., the promotion of new neuronal activity and increased production of brain cells).

Efficacy
After a full course of treatment, patients typically notice a significant and durable reduction in depressive symptoms, lasting beyond the end of the treatment course.
Approximately two-thirds of people whose depressive symptoms have not responded well to oral antidepressants will experience clinically significant improvements and symptom reduction from TMS treatment. Of these individuals, about one-half of patients receive full symptom remission, meaning that their depressive symptoms disappear completely. The other half experience relief.
Although the relief from TMS treatment is durable—meaning that it can last for many months after treatment—it is sometimes not permanent. On average, patients who undergo a full treatment course can expect their positive response to TMS treatment to last more than a year. Indeed, in one study measuring the long-term impact of TMS treatment, patients were tracked over a 12-month period, and at the end of 12 months, 2 out of 3 patients who responded to TMS treatment reported the same levels of response and benefit as they did at the end of their treatment phase. Nevertheless, patients may return for subsequent treatments (“maintenance” sessions or “reintroduction” sessions) if they feel that the benefit of their previous round of treatment has decreased.
Side Effects
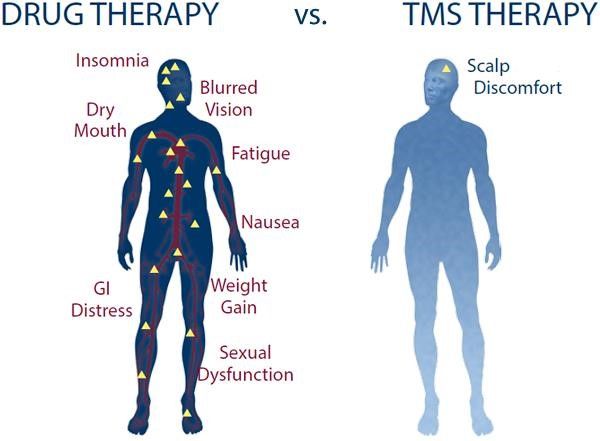
There are few side effects associated with TMS. The most common side effect reported by patients is mild discomfort at the treatment site, and possible headaches during the first week of treatment. This discomfort is typically milder than many side effects commonly associated with oral antidepressants. Indeed, TMS does not cause the common side effects associated with oral antidepressants, such as gastrointestinal upset, dry mouth, sexual dysfunction, weight gain, or sedation. A rare side effect associated with TMS is seizures. However, the risk of seizures is very low (less than 1 in 30,000), and at Core Recovery we perform a thorough assessment of each patient’s medical history to rule out risks of seizures.
Contact Us Today
If you or someone you love is struggling with depression, TMS may be able to help. Contact us today to learn more.





 In CA By O360®
In CA By O360®